Lidocaine Hydrochloride Injection USP
Overview
- Presentation(s):
-
20 mg/2 mL



50 mg/5 mL


40 mg/2 mL


20 mg/2 mL


50 mg/5 mL


300 mg/30 mL


40 mg/2 mL


100 mg/5 mL


100 mg/10 mL

200 mg/20 mL

500 mg/50 mL

200 mg/10 mL

400 mg/20 mL

1000 mg/50 mL

- NDC(s):
-
55150-158-72
55150-159-74
55150-160-72
55150-161-02
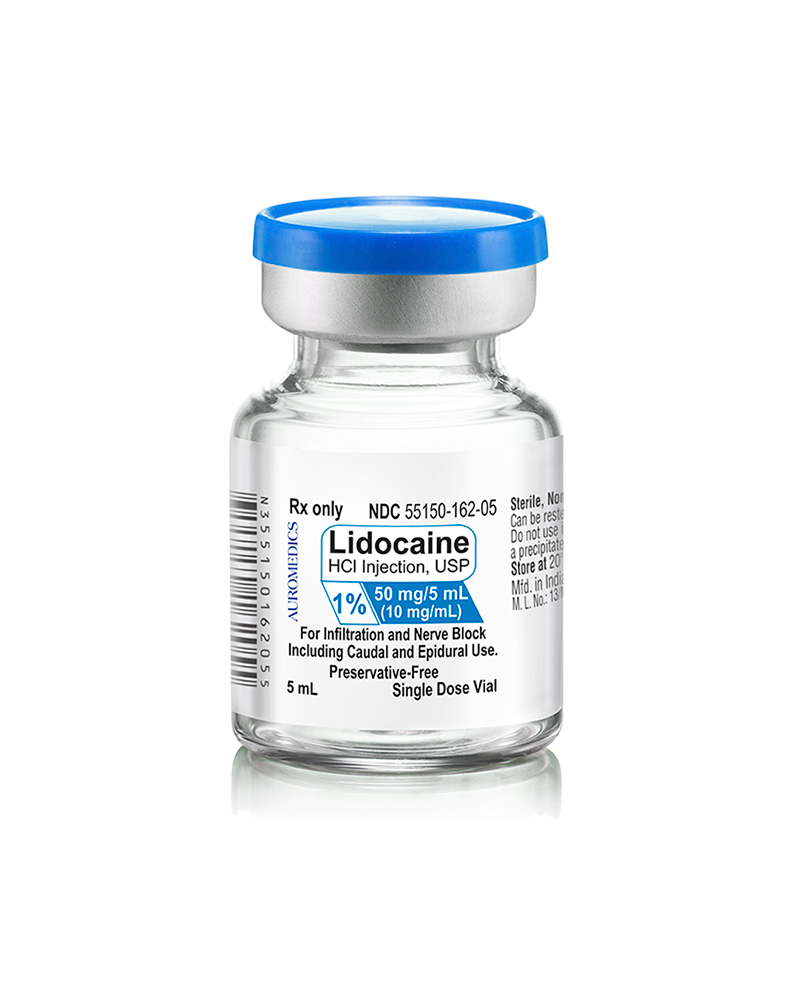
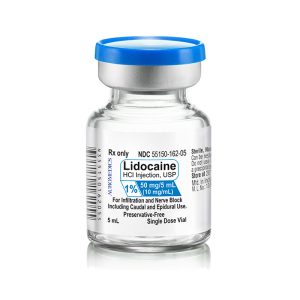
55150-162-05
55150-163-30
55150-164-02
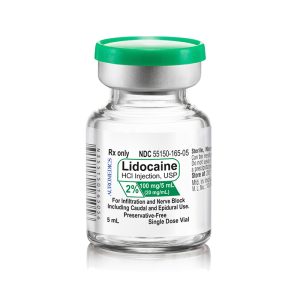
55150-165-05
55150-251-10
55150-252-20
55150-253-50
55150-254-10
55150-255-20
55150-256-50
- Reference Listed Drug:
- Xylocaine
- Therapeutic Class:
- Anesthetic
- Physical Form:
- Solution
Product Information
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-158-72 | Ampule | 20 mg/2 mL | 10 mg/mL | 2 mL | 2 mL | N/A |
Wholesaler Item Numbers
ABC
10125420Cardinal
4908281McKesson
2069193M&D
631903Ordering Information
Unit of Sale
10 ampsPack Size
10Case Quantity
30Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-159-74 | Ampule | 50 mg/5 mL | 10 mg/mL | 5 mL | 5 mL | N/A |
Wholesaler Item Numbers
ABC
10125421Cardinal
4908307McKesson
2069185M&D
631911Ordering Information
Unit of Sale
10 ampsPack Size
10Case Quantity
24Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-160-72 | Ampule | 40 mg/2 mL | 20 mg/mL | 2 mL | 2 mL | N/A |
Wholesaler Item Numbers
ABC
10125422Cardinal
4908356McKesson
2069177M&D
631879Ordering Information
Unit of Sale
10 ampsPack Size
10Case Quantity
30Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-161-02 | SD Vial | 20 mg/2 mL | 10 mg/mL | 2 mL | 2 mL | 13 mm |
Wholesaler Item Numbers
ABC
10125423Cardinal
4908299McKesson
2069169M&D
631416Ordering Information
Unit of Sale
10 vialsPack Size
10Case Quantity
30Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-162-05 | SD Vial | 50 mg/5 mL | 10 mg/mL | 5 mL | 5 mL | 20 mm |
Wholesaler Item Numbers
ABC
10125424Cardinal
4908323McKesson
2069151M&D
631481Ordering Information
Unit of Sale
10 vialsPack Size
10Case Quantity
20Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-163-30 | SD Vial | 300 mg/30 mL | 10 mg/mL | 30 mL | 30 mL | 20 mm |
Wholesaler Item Numbers
ABC
10125425Cardinal
4908331McKesson
2069128M&D
631788Ordering Information
Unit of Sale
1 vialPack Size
1Case Quantity
24Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-164-02 | SD Vial | 40 mg/2 mL | 20 mg/mL | 2 mL | 2 mL | 13 mm |
Wholesaler Item Numbers
ABC
10125426Cardinal
4908364McKesson
2069144M&D
631457Ordering Information
Unit of Sale
10 vialsPack Size
10Case Quantity
30Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-165-05 | SD Vial | 100 mg/5 mL | 20 mg/mL | 5 mL | 5 mL | 20 mm |
Wholesaler Item Numbers
ABC
10125427Cardinal
4908380McKesson
2069136M&D
631754Ordering Information
Unit of Sale
10 vialsPack Size
10Case Quantity
20Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-251-10 | MD Vial | 100 mg/10 mL | 10 mg/mL | 10 mL | 10 mL | – |
Wholesaler Item Numbers
ABC
10188248Cardinal
5460258McKesson
3910205M&D
353292Ordering Information
Unit of Sale
25 vialsPack Size
25Case Quantity
8Allergens

| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-252-20 | MD Vial | 200 mg/20 mL | 10 mg/mL | 20 mL | 20 mL | – |
Wholesaler Item Numbers
ABC
10188246Cardinal
5460266McKesson
3910197M&D
353482Ordering Information
Unit of Sale
25 vialsPack Size
25Case Quantity
4Allergens

| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-253-50 | MD Vial | 500 mg/50 mL | 10 mg/mL | 50 mL | 50 mL | – |
Wholesaler Item Numbers
ABC
10188778Cardinal
5465505McKesson
3921541M&D
384875Ordering Information
Unit of Sale
25 vialsPack Size
25Case Quantity
4Allergens

| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-254-10 | MD Vial | 200 mg/10 mL | 20 mg/mL | 10 mL | 10 mL | – |
Wholesaler Item Numbers
ABC
10188241Cardinal
5460274McKesson
3910213M&D
353656Ordering Information
Unit of Sale
25 vialsPack Size
25Case Quantity
8Allergens

| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-255-20 | MD Vial | 400 mg/20 mL | 20 mg/mL | 20 mL | 20 mL | – |
Wholesaler Item Numbers
ABC
10188244Cardinal
5460282McKesson
3910221M&D
353821Ordering Information
Unit of Sale
25 vialsPack Size
25Case Quantity
4Allergens

| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-256-50 | MD Vial | 1000 mg/50 mL | 20 mg/mL | 50 mL | 50 mL | – |
Wholesaler Item Numbers
ABC
10188777Cardinal
5465513McKesson
3921566M&D
384966Ordering Information
Unit of Sale
25 vialsPack Size
25Case Quantity
4Allergens

Additional Information
Bioequivalency Rating
APBar Code Type
LinearControlled Substance Class
N/AStorage Requirements:
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].For Package Inserts, please click on the NDC#
Our products are available from authorized distributors.
For complete ordering information, please contact your local pharmaceutical distributor.