Eptifibatide Injection
Overview
- Presentation(s):
-
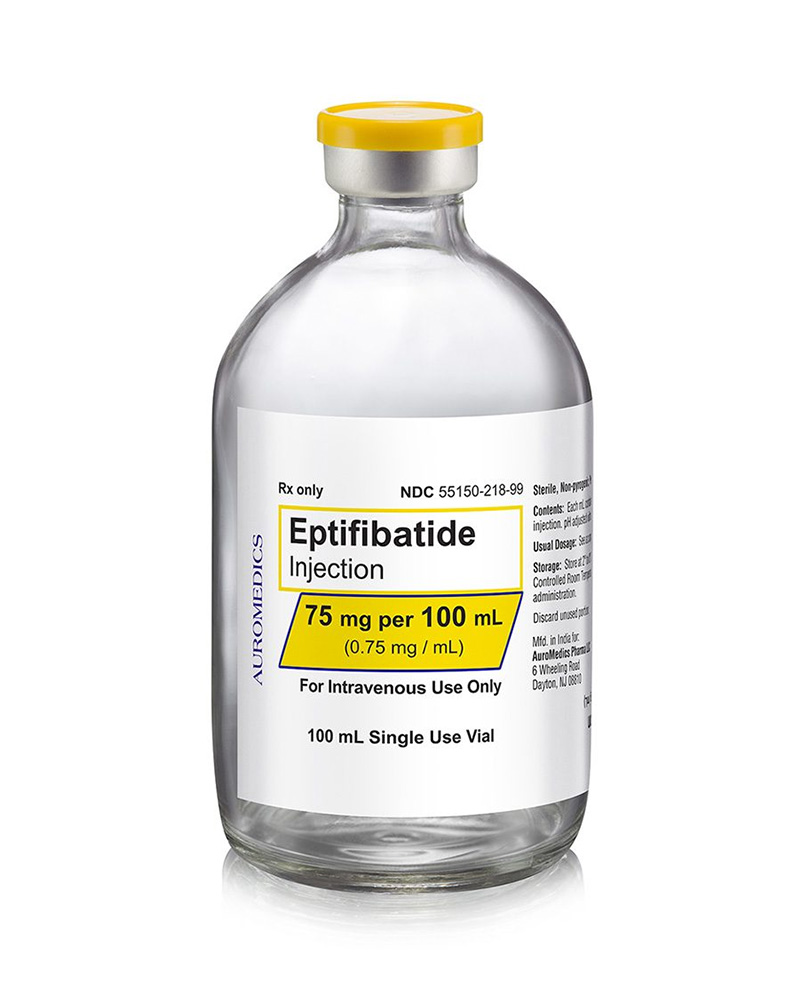
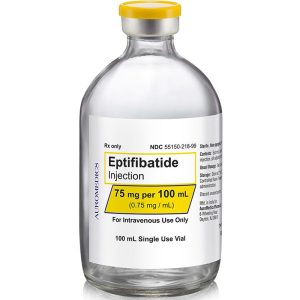
75 mg/100 mL



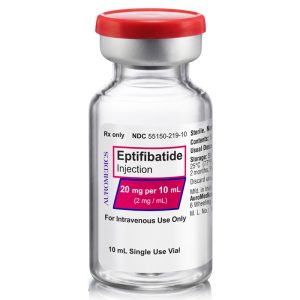
20 mg/10 mL


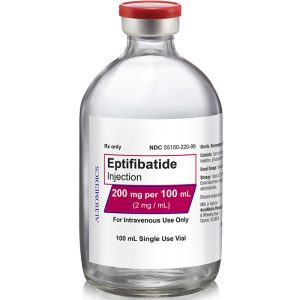
200 mg/100 mL


- NDC(s):
-
55150-218-99
55150-219-10
55150-220-99
- Reference Listed Drug:
- Integrilin®
- Therapeutic Class:
- Anticoagulant
- Physical Form:
- Solution
Product Information
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-218-99 | SD Vial | 75 mg/100 mL | 0.75 mg/mL | 100 mL | 100 mL | 20 mm |
Wholesaler Item Numbers
ABC
10161085Cardinal
5204557McKesson
3494978M&D
468603Ordering Information
Unit of Sale
1 vialPack Size
1Case Quantity
40Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-219-10 | SD Vial | 20 mg/10 mL | 2 mg/mL | 10 mL | 10 mL | 20 mm |
Wholesaler Item Numbers
ABC
10161083Cardinal
5204565McKesson
3494952M&D
468587Ordering Information
Unit of Sale
1 vialPack Size
1Case Quantity
240Allergens
| NDC | Description | Strength | Concentration | Container Size | Fill Volume | Closure |
|---|---|---|---|---|---|---|
| 55150-220-99 | SD Vial | 200 mg/100 mL | 2 mg/mL | 100 mL | 100 mL | 20 mm |
Wholesaler Item Numbers
ABC
10161084Cardinal
5204599McKesson
3494986M&D
468611Ordering Information
Unit of Sale
1 vialPack Size
1Case Quantity
40Allergens
Additional Information
Bioequivalency Rating
APBar Code Type
LinearControlled Substance Class
N/AStorage Requirements:
Store at 2° to 8°C (36° to 46°F).For Package Inserts, please click on the NDC#
Our products are available from authorized distributors.
For complete ordering information, please contact your local pharmaceutical distributor.